Lithium, recognized as the most strategically critical mineral in the global energy transition, is hailed as "white petroleum" and the "metal that drives the future." Within modern industry and the new energy system, lithium serves not only as the core raw material for power batteries and energy storage technologies but also as a fundamental resource in aerospace, ceramics/glass, and advanced materials. Its characteristics of low density, high electrochemical activity, and superior energy density position lithium ore resources as vital support for growth potential and technological breakthroughs in green energy investment.

The chemically reactive lithium element does not exist in elemental form in nature but occurs as compounds distributed in various minerals, salt lake brines, and seawater. Currently, industrially exploitable lithium resources are primarily divided into two major categories: hard-rock lithium deposits and brine lithium deposits.
Refers to deposits where lithium is enriched within silicate minerals. Ore is typically extracted via conventional open-pit or underground mining methods and then processed through crushing, grinding, and concentration to produce lithium concentrate.
【Spodumene】
Mineralogical Characteristics: Chemical formula or , with a theoretical Li2O (lithium oxide) content of 8.03%. It is one of the most important global industrial lithium mineral sources. Colors range from grey-white, green, yellow to purple, often associated with quartz, feldspar, and mica.
【Lepidolite】
Mineralogical Characteristics: Also known as lithia mica, it is a potassium lithium aluminosilicate mineral with a complex chemical structure, typically written as KLi2Al(Si4O10)(F,OH)2. It has a relatively low theoretical Li2O content, approximately 3.3% - 5.9%. It commonly occurs in granitic pegmatites, often purple or pink. Due to its complex composition and lower grade, concentration and refining are comparatively more challenging.
【Petalite】
Mineralogical Characteristics: Chemical formula Li2AlSi4O10 , with a theoretical Li2O content of 4.50%. Crystals are mostly white or colorless, with a vitreous luster and moderate floatability. Commonly found in lithium-rich pegmatites. Its processability is inferior to spodumene, but specific deposits can hold economic value.
【Typical Regions】
【Applicable Process Flows】
Primary Crushing - Secondary & Tertiary Crushing - Stage Grinding - Flotation (commonly using cationic collectors) - Thickening & Filtration - Drying; some refractory ores require combined roasting pre-treatment to enhance lithium recovery.
Refers to liquid deposits where lithium ions are enriched in surface salt lake brines, subsurface pore brines, or oilfield-associated brines. These deposits typically form in closed basins under arid to semi-arid climatic conditions, and their development heavily relies on natural evaporation and chemical extraction processes.
【Surface Salt Lake Brine】
Chemical & Compositional Characteristics: Lithium ions are present in chloride-type, sulfate-type, or carbonate-type brines. The Magnesium-to-Lithium ratio (Mg/Li) is a critical parameter impacting extraction efficiency and cost; brines with lower Mg/Li ratios are more economically valuable.
Typical Regions: Chile Salar de Atacama – Globally highest-grade surface chloride brine; Argentina Salar del Hombre Muerto – Typical sulfate-type salt lake; China Qaidam Basin – Large sulfate-magnesium subtype salt lake brine resource.
【Subsurface Pore Brine】
Chemical & Compositional Characteristics: Found within deep aquifers, lithium concentration is usually high, but extraction requires drilling, resulting in higher development costs and technical barriers.
Typical Regions: USA Clayton Valley – Representative subsurface pore brine deposit.
【Subsurface Pore Brine 】
Chemical & Compositional Characteristics: Often associated with oil and gas fields, composition is complex, frequently containing valuable elements like iodine, bromine, and potassium alongside lithium. While offering significant potential for comprehensive utilization, the refining process is intricate.
Typical Regions: China Sichuan Basin – Important oilfield-associated brine lithium resource area.
【Applicable Process Flows】
Solar evaporation in pond systems - Fractional crystallization for impurity removal (precipitating sodium salts, potassium salts, etc.) - Obtaining lithium-enriched concentrated brine (bittern) - Lithium extraction via carbonation or alkali precipitation (e.g., adding sodium carbonate to precipitate lithium carbonate) - Brines with high Mg/Li ratios or low grades may require combined adsorption, membrane separation, or solvent extraction technologies for lithium concentration and purification.
As of the end of 2024, global lithium ore production (lithium metal equivalent) was approximately 245,000 tonnes, with economically extractable reserves around 32 million tonnes. Production and reserves are highly concentrated: Australia, with 88,000 tonnes of production and 4.8 million tonnes of reserves, continues to lead hard-rock supply; Chile, with 49,000 tonnes of production and 9.3 million tonnes of reserves, holds first place in brine resources; China, with 41,000 tonnes of production and 5.28 million tonnes of reserves, ranks third. These three countries collectively account for over 76% of global production.
The South American "Lithium Triangle" (Chile, Argentina, Bolivia) holds total reserves of 15.9 million tonnes, representing half of the global total, predominantly brine resources. Meanwhile, hard-rock projects in Zimbabwe, Canada, and Brazil are ramping up production rapidly. While the USA and Bolivia possess vast reserves, they have yet to achieve large-scale production. The global lithium resource landscape is characterized by the dominance of "Australia-Chile-China" and the accelerated rise of North America, South America, and Africa.
The construction of a lithium concentrator is a complex systems engineering project involving multiple disciplines, significant investment, and lengthy timelines. It must adhere to rigorous, scientific development procedures to ensure technical feasibility, economic rationality, and environmental and social sustainability. The core construction process is as follows:

Objective:
Define ore body distribution, grade, and reserves to provide a basis for scientific decision-making.
Main Activities:
Deliverable: Mineral Resource / Ore Reserve Report
Objective:
Scientifically design an efficient, economical, and safe production line.
Main Activities:
Objective:
Ensure high-standard construction and rapid commissioning.
Main Activities:
Deliverable: Commissioned Plant (Ramp-up to Nameplate Capacity).
Objective:
Operate safely, stably, efficiently, and cost-effectively.
Main Activities:
Deliverable:
Achievement of target production metrics (Throughput, Grade, Recovery, Availability, Cost).
Objective:
Achieve rapid, secure, and low-cost value realization.
Main Activities:
Objective:
Achieve the integration of safety, environmental protection, and social responsibility.
Main Activities:
Deliverable: Safe, stable, and environmentally sound TSF operation and closure; Positive ESG performance.
The selection of lithium ore beneficiation flowsheets is highly dependent on the ore's mineralogical characteristics (e.g., mineral types, associations, liberation size). Currently applied industrial lithium ore beneficiation methods can be summarized into the following mainstream process routes, chosen based on ore type and economic viability.
Applicability:
Primarily used for concentrating spodumene from granitic pegmatites. It is the dominant global process for hard-rock lithium ores.
Basic Principle:
Based on differences in the surface physicochemical properties of spodumene compared to associated gangue minerals (like feldspar and quartz). Separation is achieved by adjusting pulp pH and adding specific reagents to render spodumene hydrophobic for flotation.
Typical Flowsheet:
ROM ore is crushed in a "Three-Stage Closed-Circuit" crushing plant to below 15mm. It is then ground in a closed-circuit ball mill/hydrocyclone circuit to a P80 of 60%-80% passing 74 microns (200 mesh) to achieve sufficient mineral liberation. Hydrocyclone desliming (removing particles finer than ~19 microns) is a key pre-treatment step. Flotation occurs in an alkaline environment (pH 8-9) using a reagent scheme of "NaOH for pH adjustment + starch as gangue depressant + oxidized paraffin soap as collector." The circuit typically employs a "Rougher-Scavenger-Cleaner" configuration with at least one rougher, two scavenger, and three cleaner stages. Concentrate undergoes thickening, filtration, and drying to produce the final product.
Advantages/Disadvantages:
Proven technology, high recovery rates, capable of producing high-grade concentrate (>5.5% Li2O); Requires tailings disposal and chemical usage, sensitive to slimes.
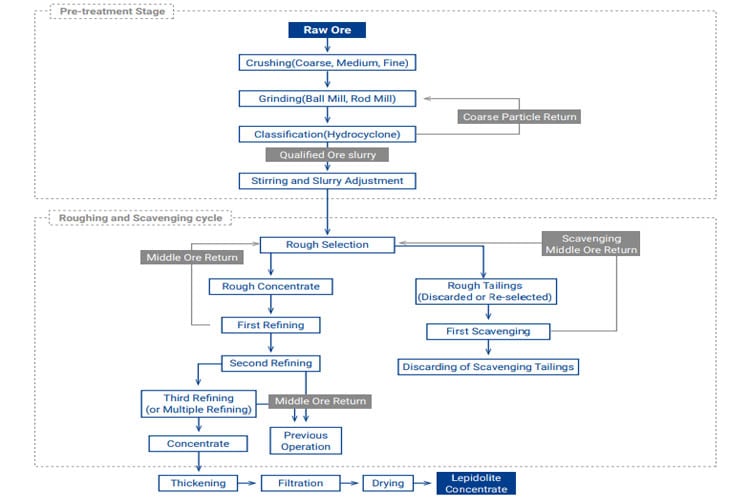
Applicability: Treating lepidolite-dominant ores, particularly suited for pegmatites in regions like Jiangxi Yichun.
Basic Principle: Utilizes cationic collectors (amine types) which selectively adsorb onto the lepidolite surface in an acidic medium, rendering it hydrophobic for flotation separation from quartz and feldspar.
Typical Flowsheet: After crushing and grinding, an "acid scrubbing" step is often added to enhance slime removal and mineral surface cleaning. Flotation occurs under acidic conditions (pH 3-4), using sulfuric acid as a modifier, sodium silicate (water glass) as a depressant, and dodecylamine or similar amines as collectors. Multi-stage circuits (e.g., one rougher, multiple scavengers, multiple cleaners) are common. Concentrate Li2O grade is typically 3.0%-4.5% and often rich in rubidium (Rb) and cesium (Cs).
Advantages/Disadvantages: Allows recovery of co-/associated rare elements; Concentrate grade is relatively low, reagent costs are high, requires high operational expertise.
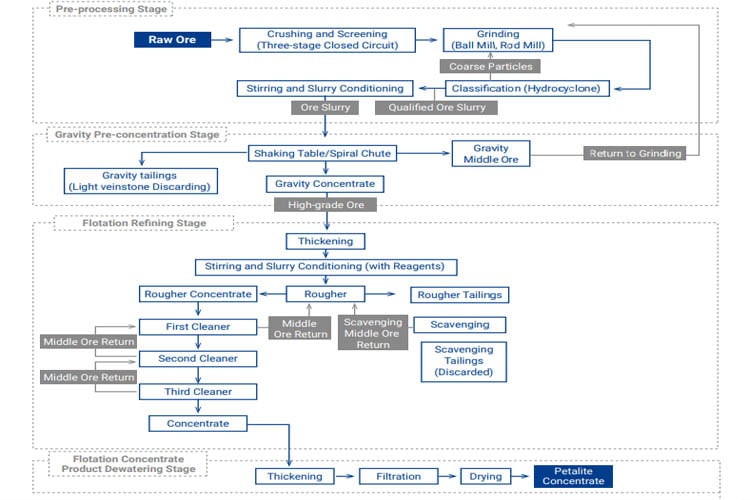
Applicability: Treating petalite ores with poor natural floatability or co-associated with heavy minerals (e.g., tantalite-columbite).
Basic Principle: Utilizes density differences for pre-concentration and waste rejection via gravity separation, followed by magnetic separation to remove iron-bearing minerals, and finally flotation for purification.
Typical Flowsheet: ROM ore is ground to a medium fineness. Gravity separation using jigs or shaking tables rejects large volumes of low-density gangue and provides a preliminary concentrate enriched in petalite and heavy minerals. The gravity concentrate undergoes high-intensity magnetic separation (HIMS) to remove iron impurities. The non-magnetic fraction is reground and floated (often using cationic or combined cationic/anionic collectors) to further remove silicate gangue.
Advantages/Disadvantages: Enables comprehensive recovery of multiple valuable minerals; Flowsheet is long and costly, operation and control are complex.
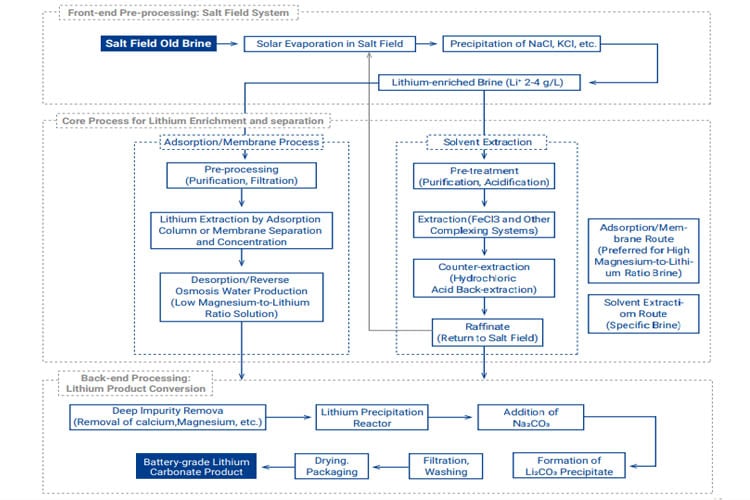
Applicability: Extracting lithium from salt lake or subsurface brines with high lithium ion content. A major pathway for current lithium resource development.
Basic Principle: Utilizes solar evaporation ponds for lithium ion concentration, followed by chemical precipitation, adsorption, membrane separation, or solvent extraction to separate lithium from other ions (e.g., Mg, Na), ultimately producing lithium salt products.
Typical Flowsheet: Brine undergoes multi-stage evaporation in solar ponds, precipitating sodium and potassium salts sequentially, yielding concentrated lithium-rich brine (bittern). The bittern is purified to remove impurities (e.g., Mg, Ca, B). Sodium carbonate is then added to precipitate crude lithium carbonate. Further purification steps (dissolution, impurity removal, recrystallization) yield battery-grade product. Brines with high Mg/Li ratios often require pre-concentration steps like adsorption or membrane technologies.
Advantages/Disadvantages: Lower operating costs, high product purity, large resource scale; Long development timelines, severely constrained by climate and geography, high environmental compliance requirements.
The value realization of lithium ore beneficiation aims to transform ROM ore into higher-value lithium products suitable for direct use in downstream industries through physical or chemical concentration. Core pathways include concentrate sales, lithium salt conversion, and tolling arrangements, ultimately driven by the lithium demand from the new energy battery sector.
The concentrator processes ROM ore through crushing, grinding, flotation, etc., and directly sells the lithium concentrate product (e.g., 5%-6% Li2O spodumene concentrate). Pricing is typically linked to international lithium chemical prices, net of subsequent conversion costs. This approach offers rapid capital turnover, avoids investment and operational risks associated with the lithium conversion stage, and is suitable for companies with good resources but limited capital or those focused solely on concentration.
Through an integrated concentration-conversion flow, self-produced or purchased concentrate is further processed into battery-grade lithium salts like lithium carbonate or lithium hydroxide. Products are sold directly based on spot market or long-term contract pricing, offering stronger pricing power and larger profit margins. Although this model requires significant upfront investment and higher technical capability, it enhances resilience against concentrate price fluctuations and improves overall profitability and risk management across the value chain.
The concentrating company sends its self-produced concentrate to a third-party converter (toll processor). The processor converts the concentrate into lithium salts for a fee (tolling charge), and the salts are returned to the concentrator company for sale. This approach allows the company to retain product ownership, offering flexibility in sales timing and partners, while avoiding heavy investment in conversion assets and capturing the lithium salt market premium. It is suitable for companies with concentrate production capacity but without integrated conversion facilities. To enhance overall value, companies can also comprehensively recover valuable associated elements like rubidium (Rb), cesium (Cs), and tantalum (Ta) present in lithium ores, broadening revenue streams. Furthermore, optimizing concentrate recovery rates, reducing reagent and energy consumption costs, implementing advanced process control (APC), and ensuring compliance management can enhance profitability while ensuring stable production. Value is ultimately realized through the rigid demand for lithium materials in the new energy battery sector.




Learn proven ways to reduce crushing plant operating costs, including equipment selection, energy efficiency, wear part control, and process optimization to lower cost per ton and improve ROI.

This article will explore 10 prominent uses of dolomite, shedding light on its versatility and highlighting its importance in today’s global economy.

Discover the cost of portable crushing plants in Tanzania. Learn factors affecting price, capacity, mobility options, and how to choose the right solution for your mining or quarry project.
Fill your requirements here, and we'll send the custmized solution and quotation to you by the reserved contact information.